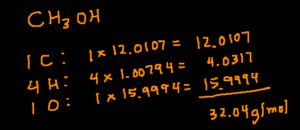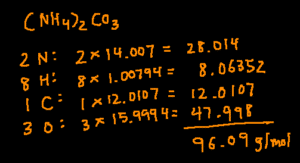How to Calculate Molar Mass of Anything. Step by Step Explanation with Multiple Examples
What is molar mass?
Lets start by talking what the term means. Simply said, molar mass is how much one mole of a substance weighs. That substance can be an element or a compound.
How to calculate molar mass with examples
We’ll go through three examples progressing from easy to “difficult”. By the end of this post, you’ll be able to calculate the molar mass of anything.
Example #1: Single element
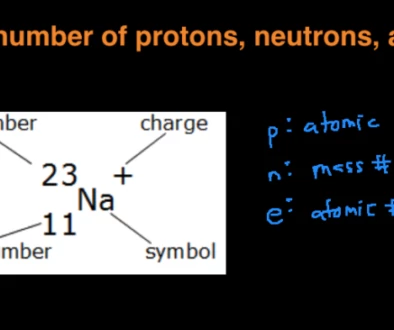
Sodium (Na)
Finding the molar mass of a single element is really simple. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or g/mol.
 From this, you can see that sodium’s molar mass will be 22.99 g/mol.
From this, you can see that sodium’s molar mass will be 22.99 g/mol.
Example #2: Simple compound
CH3OH
Start by determining how many of each elements there are by looking the subscripts (small number next to the element symbol). In this compound, there are 1 C, 4 H (3+1), and 1 O. Next, multiply the number of a particular element by its molar mass. Finally, add the products together and you’ll arrive at the answer.
Example #3: “Complicated” compound
(NH4)2CO3
The process is very similar to calculating the molar mass of a simple compound. The only difference is you’ll need to multiply the subscript on the outside of the parenthesis by the subscripts inside the parenthesis.
Start by determining how many of each elements there are. In this compound, there 2N, 8H, 1C, and 3O. Next, multiply the number of a particular element by its molar mass. Finally, add the products together and you’ll arrive at the answer.
After reading and working through these three examples, you should be able to calculate the molar mass of anything.
Additional Resources
This molar mass calculator allows you to double check if you’re getting the right answer.