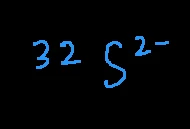How to Find the Number of Protons, Neutrons, and Electrons. Step by Step Explanation with Examples
In this post, we’ll be going over how to determine the number of protons, neutrons, and electrons in an atom or ion.
We’ll first start by discussing what each of the components in the nuclide notation means. Then we’ll go through two examples together.
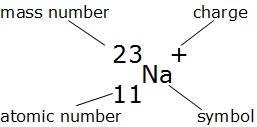
The Nuclide Notation
The letter(s) in the middle is the symbol of the element.
The number on the bottom left corner is the atomic number, which tells you the number of protons.
The number on the upper left corner is the mass number, which is equal to the neutrons and protons added together.
Lastly, the charge is on the upper right corner. If there isn’t any number or signs, then it means that atom has no charge and is neutral.
Rules to Finding Number of Protons, Neutrons, and Electrons
# of protons = atomic number
# of neutrons = mass number – atomic number
# of electrons = atomic number – charge
That’s it!
Examples
Great, lets apply the rules to some examples.
# of protons = 17
# of neutrons = 37 – 17 = 20
# of electrons = 17 – 0 = 17
# of protons = 16 (the atomic number is not given, but can be found on the periodic table)
# of neutrons = 32 – 16 = 16
# of electrons = 16 – (-2) = 18
Additional Practice
Try these on your own and check the answer below
- 78Se2-
- 39K+
ANSWERS
- 34 protons, 44 neutrons, 36 electron
- 19 protons, 20 neutrons, 18 electron
Additional Resources
If you have any questions, leave a comment below.